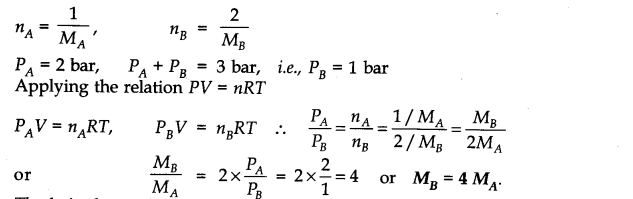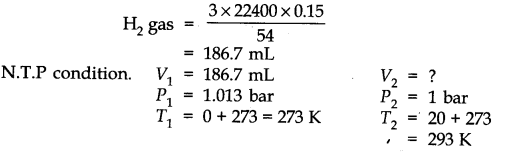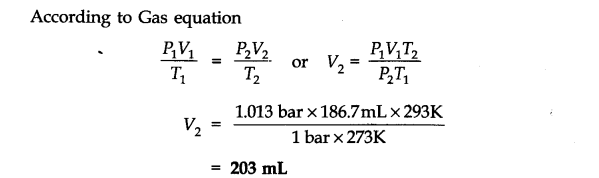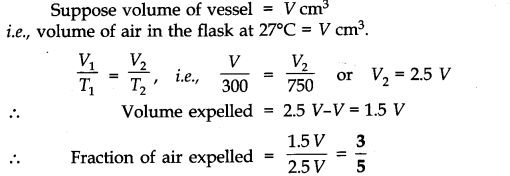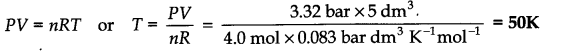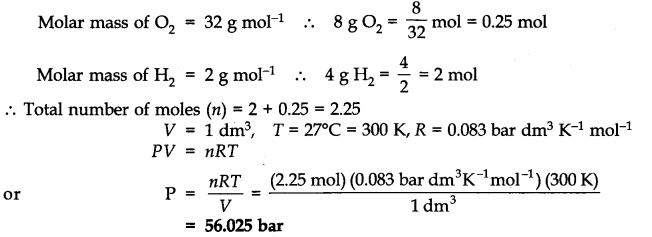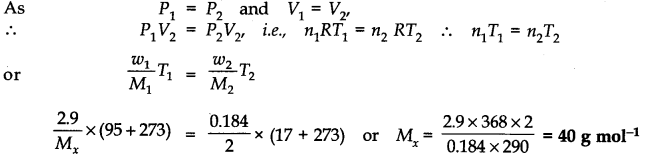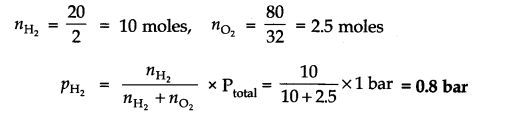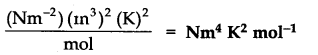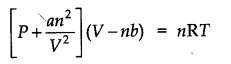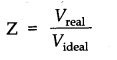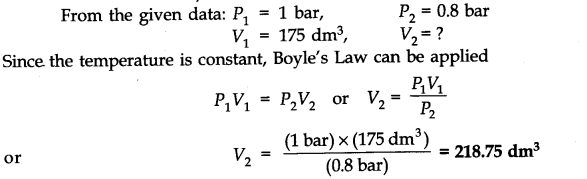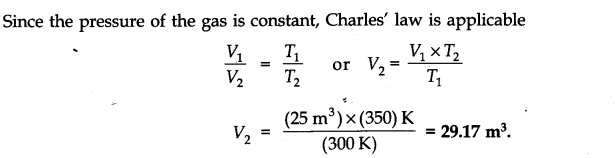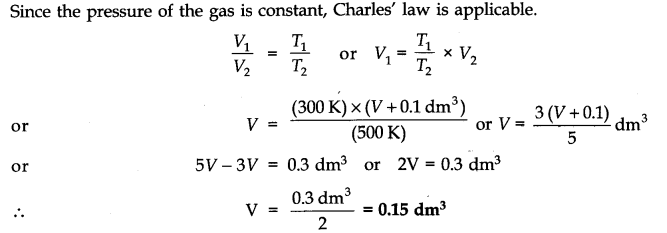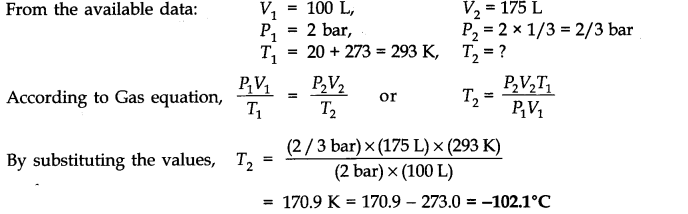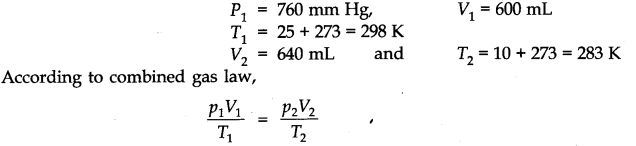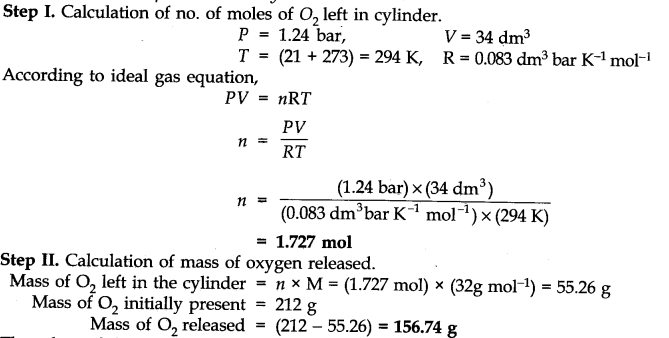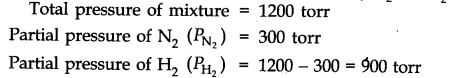NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction: NCERT which stands for the National Council Of Educational Research and Training is responsible for designing and publishing textbooks for all the classes and subjects. NCERT textbooks cover all the topics and apply to the Central Board Of Secondary Education (CBSE) and various state boards.
We provide free NCERT solutions in Hindi medium as well as English Medium for all the classes. Created by subject matter experts, these NCERT Solutions in Hindi are very helpful to students of all classes.
The NCERT Solutions offer insight into the exam pattern, the latest syllabus, and the complete marking scheme. The solutions are available in the pdf format which can be easily downloaded and saved for future reference as well. We are providing you a free PDF of NCERT solutions for class 11 Chemistry chapter 8 Redox reactions for download.
NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction
The chemistry Class 11 Ncert Solutions Chapter 8 Redox Reaction deals with the permutations and combinations between the oxidation and reduction reactions. There are a number of phenomena, biological as well as physical that is concerned with redox reactions.
Also, reactions find their use in biological, pharmaceutical, metallurgical, industrial, and agricultural areas. Therefore, to guide you in your preparation for NCERT Class 11, we have offered the downloadable PDF for NCERT solutions for Class 11 Chemistry Chapter 8.
This chapter is solved by our subject matter expert and prepared as per the NCERT guidelines. This PDF consists of the exercise questions along with solutions that can help you revise the complete syllabus and thereby help you score higher marks.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction from below.
NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction
What will you learn in CBSE Class 11 Chemistry Chapter 8 Redox Reaction?
After studying our 11th Class Chemistry Chapter 8 Solutions pdf students will be able to identify the redox reactions as a reaction in which reduction and oxidation reactions are occurring simultaneously. Also, students will be defining the terms reduction, oxidation, oxidant, and reductant. Additionally, students will explain the mechanism of redox reactions using the electron transfer process.
Along with that, you will use the concept of oxidation number in identifying reductants and oxidation in a reaction. So, you learn to classify the redox reaction into combination, displacement, decomposition, and disproportionation. Adding to that, you will suggest a comparative order among the various oxidants and reductants. You will also learn about balancing the chemical equations using the half-reaction method and oxidation number.
Whether you are unable to solve the difficult problems in the NCERT textbook or you are facing difficulty in understanding the complex theories of Science, Download class 11 chemistry chapter 8 NCERT solutions pdf to resolve all your doubts.
NCERT Solutions is a wholesome source of study material that provides the student with access to solved queries and information. If you practice the solutions on a regular basis, you will give yourself a better chance of scoring higher points in your exam. With its help, students will be able to learn easy methods and ways to understand difficult concepts of redox reactions and oxidation reactions.
Furthermore, they will also get to study competitive electron transfer reactions, electron transfer reach, oxidation numbers, types of redox reactions, and balancing the redox reactions. The chemistry chapter 8 redox reactions are a part of unit 8. In the final exam, unit 8, 9, 10, and 11 holds a total weight of 16 marks.
Below are the subtopics given in this chapter.
- Ex 8.1 – Classical Idea of Redox Reactions – Oxidation and Reduction Reactions
- Ex 8.2 – Redox Reactions in terms of Electron Transfer Reactions
- Ex 8.2.1 – Competitive Electron Transfer Reactions
- Ex 8.3 – Oxidation Number
- Ex 8.3.1 – Types of Redox Reactions
- Ex 8.3.2 – Balancing of Redox Reactions
- Ex 8.3.3 – Redox Reactions as the Basis for Titrations
- Ex 8.3.4 – Limitations of Concept of Oxidation Number
- Ex 8.4 – Redox Reaction and Electrode Process
Advantages of NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction
Chemistry is one of the challenging subjects for most of the students. It is equally important because in Class 11th students prepare a foundation that will be of immense help in Class 12th. With the right study material and proper exam preparation, students can score very good marks on their board exams.
- NCERT Solution for Class 11 Chemistry Chapter 8 covers all the important topics given in the chapter.
- It will give you an idea about the marking scheme, pattern, and questions and prepare you for your exam.
- Solutions will help you build your performance before the actual exam.
- Practicing solutions will help you increase your pace and manage time during real exams.
- NCERT solutions are framed by extracting content from the NCERT textbook.
- The diagrams used are neatly labeled and self-explanatory
- Conceptual-based learning promoted
- Answering methodologies as per the expected pattern
- Solutions developed by subject experts
Access NCERT Solutions For Class 11 Chemistry Chapter 8
Question 1. What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30°C?
Answer: P1 = 1 bar,P2 = ? V1= 500 dm3 ,V2=200 dm3
As temperature remains constant at 30°C,
P1V1=P2V2
1 bar x 500 dm3 = P2 x 200 dm3 or P2=500/200 bar=2.5 bar
Question 2. A vessel of 120 mL capacity contains a certain amount of gas at 35°C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180 mL at 35°C. What would be its pressure?
Answer: V1= 120 mL, P1=1.2 bar,
V2 = 180 mL, P2 =?
As temperature remains constant, P1V1 = P2V2
(1.2 bar) (120 mL) = P2 (180mL)
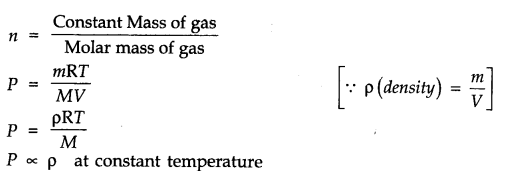
Question 3. Using the equation of state PV = nRT, show that at a given temperature, density of a gas is proportional to the gas pressure P.
Answer: According to the ideal gas equation
PV = nRT or PV=nRT/V
Question 4. At 0°C, the density of a gaseous oxide at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Answer: Using the expression, d =MP/RT , at the same temperature and for same density,
M1P1 = M2P2 (as R is constant)
(Gaseous oxide) (N2)
or
M1 x 2 = 28 x 5(Molecular mass of N2 = 28 u)
or M1 = 70u
Question 5. The pressure of l g of an ideal gas A at 27°C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask at the same temperature, the pressure becomes 3 bar. Find the relationship between their molecular masses.
Answer: Suppose molecular masses of A and B are MA and MB respectively. Then the number of moles will be
Question 6. The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20 °C and one bar will be released when 0.15g of aluminum reacts?
Answer: The chemical equation for the reaction is
2 Al + 2 NaOH + H20 -> 2 NaAl02 + 3H2 (3 x 22400 mL At N.T.P)
2 x 27 = 54 g.
54 g of Al at N.T.P release
H2 gas = 3 x 22400 0.15 g of Al at N.T.P release
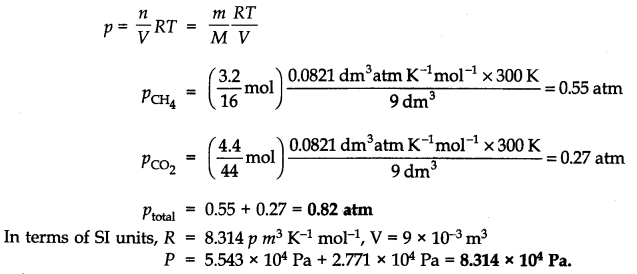
Question 7. What will be the pressure exerted by a mixture of 3.2g of methane and 4.4g of carbon dioxide contained in a 9 dm3 flask at 27 °C?
Answer:
Question 8. What will be the pressure of the gas mixture when 0.5 L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in all vessel at 27 °C?
Answer: Calculation of partial pressure of H2 in 1L vessel P1= 0.8 bar,
P2= ? V1= 0.5 L , V2 = 1.0 L
As temperature remains constant, P1V1 = P2V2
(0.8 bar) (0.5 L) = P2 (1.0 L) or P2 = 0.40 bar, i.e., PH2 = 0.40 bar
Calculation of partial pressure of 02 in 1 L vessel
P1‘ V1 = P2‘V2‘
(0.7 bar) (2.0 L) = P2 (1L) or P2‘ = 1.4 bar, i.e.,Po2= 1.4 bar
Total pressure =PHz + PQ2 = 0.4 bar + 1.4 bar = 1.8 bar
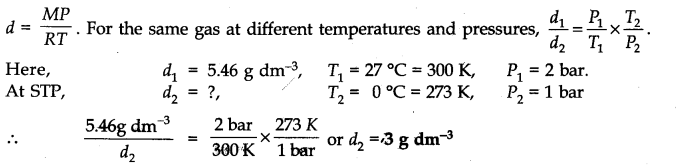
Question 9. Density of a gas is found to be 5.46 g/dm3 at 27 °C and at 2 bar pressure. What will be its density at STP?
Answer:
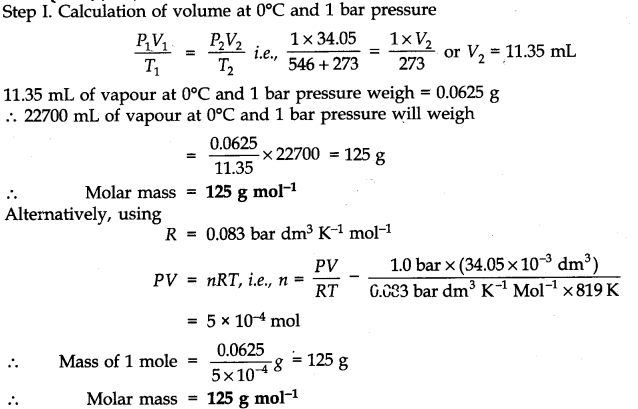
Question 10. 34.05 mL of phosphorus vapor weighs 0.0625 g at 546°C and 1.0 bar pressure. What is the molar mass of phosphorus?
Answer:
Question 11. A student forgot to add the reaction mixture to the round bottomed flask at 27 °C but instead, he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer, he found the temperature of the flask was 477 °C. What fraction of air would have been expelled out?
Answer:
Question 12.Calculate the temperature of 4.0 moles of a gas occupying 5 dm3 at 3.32 bar (R = 0.083 bar dm3 K-1 mol-1)
Answer:

Question 13. Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Answer: Molecular mass of N2 = 28g
28 g of N2 has No. of molecules = 6.022 x 1023 1.4 g of
N2 has No. of molecules = 6.022 x 1023 x 1.4 g/28 g
= 3.011 x 1022 molecules.
Atomic No. of Nitrogen (N) = 7
1 molecule of N2 has electrons = 7 x 2 = 14
3.011 x 1022 molecules of N2 have electrons
= 14 x 3.011 x 1022
= 4.215 x 1023 electrons.
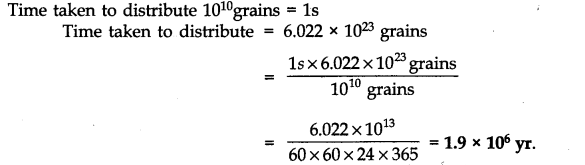
Question 14. How much time would it take to distribute one Avogadro number of wheat grains if 1010 grains are distributed each second ?
Answer:
Question 15. Calculate the total pressure in a mixture of 8g of oxygen and 4g of hydrogen confined in a vessel of l dm3 at 27°C. R = 0.083 bar dm3 K-1 mol-1.
Answer:
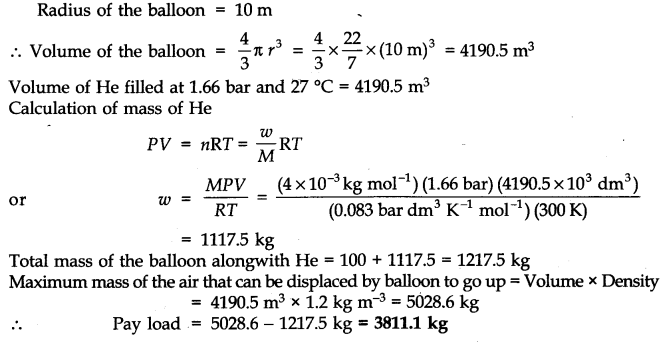
Question 16. Payload is defined as the difference between the mass of the displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at 27°C (Density of air = 1.2 kg m-3 and R = 0.083 bar dm3 K-1 mol-1).
Answer:
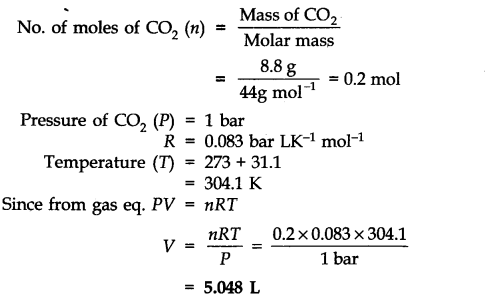
Question 17. Calculate the volume occupied by 8.8 g of CO2 at 31.1 °C and 1 bar pressure. R = 0.083 bar LK-1 mol-1
Answer:
Question 18. 2.9 g of a gas at 95°C occupied the same volume as 0.184 g of hydrogen at 17°C at the same pressure. What is the molar mass of the gas ?
Answer:
Question 19. A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
Answer: As the mixture H2 and O2 contains 20% by weight of dihydrogen, therefore, if H2 = 20g, then O2 = 80g
Question 20. What would be the SI unit for the quantity PV2T2/n?
Answer:
Question 21. In terms of Charles’ law explain why -273°C is the lowest possible temperature.
Answer: At -273°C, volume of the gas becomes equal to zero, i.e., the gas ceases to exist.
Question 22. Critical temperature for CO2 and CH4 are 31.1°C and -81.9°C respectively. Which of these has stronger intermolecular forces and why?
Answer: Higher the critical temperature, more easily the gas can be liquefied, i.e., greater are the intermolecular forces of attraction. Hence, Co2 has stronger intermolecular forces than CH4.
Question 23. Explain the physical significance of vander Waals parameters.
Answer: ‘a’ is a pleasure of the magnitude of the intermolecular forces of attraction, while b is a measure of the effective size of the gas molecules.
MORE QUESTIONS SOLVED
I. Very Short Answer Type Questions
Question 1. What is the value of the gas constant in SI units?
Answer: 8.314 JK-1 mol-1.
Question 2. Define boiling point of a liquid.
Answer: The temperature at which the vapor pressure of a liquid is equal to external pressure is called boiling point of the liquid.
Question 3. What is SI unit of (i) Viscosity (ii) Surface tension?
Answer: (i) Unit of viscosity is Nsm-2
(ii) Unit of surface tension is Nm-1
Question 4. What is the effect of temperature on (i) surface tension and (ii) Viscosity?
Answer: (i) Surface tension decreases with the increase of temperature.
(ii) Viscosity decreases with the increase of temperature.
Question 5. What is the unit of coefficient of viscosity?
Ans. Poise.
Question 6. What do you understand by the laminar flow of a liquid?
Answer: The type of flow in which there is a regular gradation of velocity in passing from one layer to the next is called laminar flow.
Question 7. What do you mean by compressibility factor?
Answer: The deviation from ideal behavior can be measured in terms of compressibility factor Z.
Z=PV/nRT
Question 8. What is Boyle Temperature?
Answer: The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure, is called Boyle temperature or Boyle point.
Question 9. What is meant by elastic collision ?
Answer: Collision in which there is no loss of kinetic energy but there is transfer of energy, is called elastic collision.
Question 10. Define critical temperature of gas.
Answer: The temperature above which a gas cannot be liquefied.
Question 11. What are real gases ?
Answer: A gas which can deviate from ideal gas behaviour at higher pressure and lower temperature, is called a real gas.
Question 12. Define an ideal gas.
Answer: A gas that follows Boyle’s law, Charles’ law and Avogadro law strictly, is called an ideal gas.
Question 13. Name four properties of gases.
Answer:
- Gases, have no definite shape and no definite volume.
- There is no force of attraction existing between the molecules of gases.
- Gases are highly compressible.
- Gases can mix evenly and can spread in a whole space.
Question 14. State Dalton’s law of partial pressure.
Answer: Daltons’ Law states that the total pressure exerted by the mixture of non-reactive gases is equal to the sum of the partial pressures of individual gases.
Question 15. What do you mean by aqueous tension?
Answer: Pressure exerted by saturated water vapor is called aqueous tension.
Question 16. Give the mathematical expression for the ideal gas equation.
Answer: PV = nRT
Where R is called the Gas constant.
Question 17. Write the van der Waals equation for n moles of a gas.
Answer:
Where ‘a’ and ‘V is van der Walls constants.
Question 18. How is the compressibility factor expressed in terms of the molar volume of the real gas and that of the ideal gas?
Answer:
Question 19. Why do liquids diffuse slowly as compared to gases?
Answer: In liquids, the molecules are more compact in comparison to gases.
Question 20. What is the effect of temperatures on the vapor pressure of a liquid?
Answer: Vapour pressure increases with rise in temperature.
Question 21. Why falling liquid drops are spherical?
Answer: Because of the property of surface tension, liquid tends to minimize its area.
II. Short Answer Type Questions
Question 1. A weather balloon has a volume of 175 dm3 when filled with hydrogen gas at a pressure of 1.0 bar. Calculate the volume of the balloon when it rises to a height where the atmospheric pressure is 0.8 bar. Assume that temperature is constant.
Answer:
Question 2. A certain amount of a gas at 27°C and 1 bar pressure occupies a volume of 25 m3. If the pressure is kept constant and the temperature is raised to 77°C, what will be the volume of the gas?
Answer: From the available data: V1 = 25 m3, T1= 27 + 273 = 300 K
V2 = ? T2 = 77 + 273 = 350 K
Question 3. A flask was heated from 27°C to 227°C at constant pressure. Calculate the volume of the flask if 0.1 dm3 of air measured at 227°C was expelled from the flask.
Answer: Let the volume of the flask = V dm3 (after expelling the air)
V1 = V dm3, T1 = 27 + 273 = 300K
VT2 = (V + 0.1) dm3, T2 = 227 + 273 = 500 K
Question 4. A gas occupying a volume of 100 liters is at 20°C under a pressure of 2 bar. What temperature will it have when it is placed in an evacuated chamber of volume 175 liters? The pressure of the gas in the chamber is one-third of its initial pressure.
Answer:
Question 5. At 25°C and 760 mm of Hg pressure, a gas occupies 600 mL volume. What will be its pressure at a height where the temperature is 10°C and the volume of the gas is 640 mL?
Answer:
Question 6. A 34.0 dm3 cylinder contains 212 g of oxygen gas at 21°C. What mass of oxygen must be released to reduce the pressure in the cylinder to 1.24 bar?
Answer:
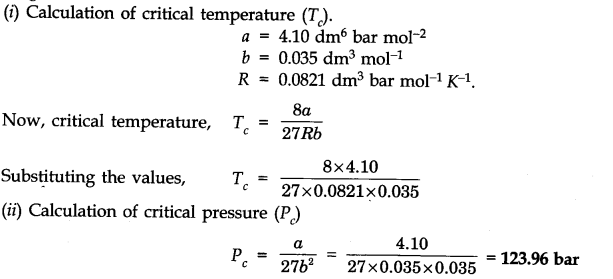
Question 7. The values of van der Waal’s constants for a gas are a = 4.10 dm6 bar mol-2 and b = 0.035 dm3 bar mol-1. Calculate the values of the critical temperature and critical pressure for the gas.
Answer:
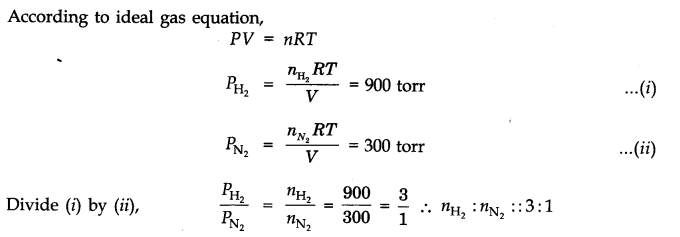
Question 8. The pressure of a mixture of H2 and N2 in a container is 1200 torr. The partial pressure of nitrogen in the mixture is 300 torr. What is the ratio of H2 and N2 molecules in the mixture?
Answer:
Question 9. (a) What do you mean by Surface Tension of a liquid?
(b) Explain the factors which can affect the surface tension of a liquid.
Answer: (a) Surface tension: It is defined as the force acting per unit length perpendicular to the line drawn on the surface. Its unit is Nnr1.
(b) Surface tension of a liquid depends upon the following factors.
(i) Temperature: Surface tension decreases with a rise in temperature. As the temperature of the liquid increases, the average kinetic energy of the molecules increases. Thus, there is a decrease in the intermolecular force of attraction which decreases the surface tension.
(ii) Nature of the liquid: Greater the magnitude of intermolecular forces of attraction in the liquid, the greater will be the value of surface tension.
We have covered the complete guide on CBSE NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction. Feel free to ask us any questions in the comment section below.
FAQ: NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction
Can I download NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction PDF for free?
Yes, you can download NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reaction PDF for free.
What are redox reactions in Class 11?
The Redox reaction is an oxidation-reduction chemical reaction where the reactants have a change in their oxidation states.
What is the weightage of Chapter 8 Class 1 Chemistry?
The chemistry chapter 8 redox reactions are a part of unit 8. In the final exam, unit 8, 9, 10, and 11 holds a total weight of 16 marks.
What are the topics taught in Class 11 Chapter 8 Chemistry?
You can refer to the above article to get the details of the topics taught in Class 11 Chapter 8 Chemistry.