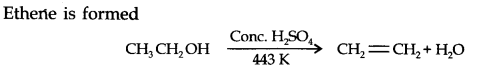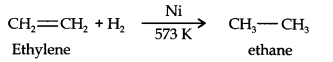NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons: NCERT solutions help students understand complex concepts in a better way. NCERT Solutions for Class 11 Chemistry Chapter 13 is Hydrocarbons. And NCERT solutions include all the topics in the chapter with in-depth explanations for the same. So, going through the solutions will help you to know how to formulate answers in a better way.
It also helps revise the entire chapter so that you are thorough with all the concepts. NCERT Solutions not only help you to prepare for NCERT Solutions for Class 11 Chemistry Chapter 13 final exams but also the board exams of Class 12, as well as other competitive exams such as NEET and IIT-JEE.
NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
Experts having immense knowledge in Chemistry develop NCERT Solutions for Class 11 Chemistry Chapter 13 with the sole intent of offering rich educational content for students of Class 11.
The chapter, Hydrocarbons, consists of six main topics. Each of these six topics further consists of sub-topics related to a concept.
NCERT solutions are available for download in PDF format. So, you can download and start studying from them right away. Also, for all those students who are not well-versed in English, you can download the Hindi versions of NCERT solutions.
The solutions also have been solved as well as practice questions at the end of the chapter. So, you can prepare for the exams by working on them. And in case you are not able to figure out the answer to any of the questions, you can always refer to the solutions. The solutions also give you hints, tips, and other tricks that help you to write your answers in a faster and more effective way.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons from below.
Download NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2020/09/chapter_13_hydrocarbons.pdf”, “#example1”);
What will you learn in NCERT Class 11 Chemistry Chapter 13 Hydrocarbons?
The reaction of Carbon and Hydrogen forms hydrocarbons. If a hydrocarbon has single bonds, then it is saturated. And if it has multiple bonds, it is unsaturated.
Also, those hydrocarbons formed by Benzene and its derivatives are known as aromatic hydrocarbons.
Acyclic and Heterocyclic Hydrocarbons
Sometimes, hydrocarbons consist of only carbon compounds. And such compounds are known as acyclic hydrocarbon compounds. Similarly, hydrocarbon compounds having a carbon atom along with other elements are known as heterocyclic hydrocarbon compounds.
Alkanes
Alkanes are the simplest forms of hydrocarbons. In these types of compounds, the carbon atoms have single covalent bonds that are fully saturated by Hydrogen atoms. So, they are also known as saturated hydrocarbons.
Alkanes have strong bonds and are, therefore, chemically inert. And because of this, they also are known as ‘paraffin,’ meaning that they have a lesser affinity towards chemical reactions.
Nomenclature of Alkanes
The IUPAC has guidelines for writing the structure of Hydrocarbons. As such, you can use the following steps to write the structural formula for different hydrocarbon compounds.
- Find the longest chain that is present in the hydrocarbon compound.
- Number the chain from left to right.
- Find the alkyl group.
- Write the IUPAC name.
When writing the formula, separate each number by a comma. Also, isolate the numbers from the names. You don’t have to write the prefixes if you are using their alphabet substitutes.
Alkynes
Alkynes are those hydrocarbons with three bonds in the molecules. Acetylenes are the most important compounds in this class of hydrocarbons.
Nomenclature of Alkynes
You can give names to alkynes using the parent alkanes and add the suffix ‘-yne’ to them. You must then assign numbers to the carbon chain along with the triple bonds, starting from the end that is nearest to that bond.
Isomerism
There are two types of isomerism when it comes to organic compounds. The first type is structural isomerism, and the second type is geometrical isomerism. Most hydrocarbons don’t show any signs of structural isomerism. However, some compounds that have double bonds tend to show geometrical isomerism.
Aromaticity
Hydrocarbons exhibit aromaticity if they satisfy any of the following conditions.
- The hydrocarbons must be cyclic and planar.
- Each atom in the hydrocarbon must have a parallel p orbital so that they can overlap continuously.
Physical and Chemical Properties of Benzene
Benzene is a colorless liquid and is insoluble in water. However, it is soluble in substances like alcohol, chloroform, and ether.
It is also a suitable solvent for various other organic and inorganic substances like fat, resins, and Sulphur. Additionally, Benzene also undergoes Electrophilic Substitution and Addition Reactions.
Benefits of Studying from NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
Studying Chemistry from NCERT solutions come with a ton of benefits, especially for a concept like hydrocarbons. For instance, the solutions have practice questions at the end of each chapter.
So, students can work on them and come out on top in not only the class 11 exams but also in the class 12 board exams and various other competitive examinations. Likewise, there are other advantages to studying NCERT solutions.
1. Exemplar Problems
The solutions come with exemplar problems that offer a means for the students to understand the subject from a unique perspective.
2. Tips and Tricks
The solutions offer tips and tricks on the way the students should be writing answers in the exams. These tips and tricks should help them score well for each question as well as in the subject.
3. Worksheets
The solutions also have worksheets and many questions for the students to answer. By working on them, you can rest assured that you will score well in the exams.
4. Numerical Problems
In addition to worksheets and exercise questions, NCERT solutions for Chemistry also have numerical problems that are likely to appear in the exams. Their question paper can consist of various levels of numerical problems, and NCERT solutions list all of them.
It is best if you studied prominent topics such as Hydrocarbons by referring to NCERT solutions. They not only give you in-depth insights about the chapter but also help you to prepare for the exams by offering questions and problems at the end of each chapter.
Access NCERT Solutions For Class 11 Chemistry Chapter 13
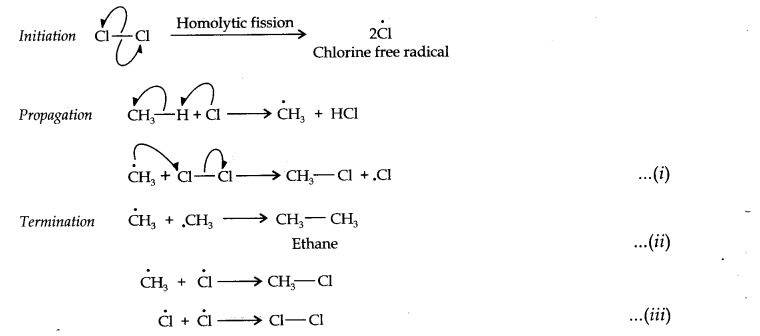
Question 1. How do you account for the formation of ethane during the chlorination of methane?
Answer: Chlorination of methane is a free radical reaction that occurs by the following mechanism:
From the above mechanism, it is evident that during the propagation step, CH3 free radicals are produced which may undergo three reactions, i.e., (i) — (iii). In the chain termination step, the two CH3 free radicals combine together to form an ethane (CH3—CH3) molecule.
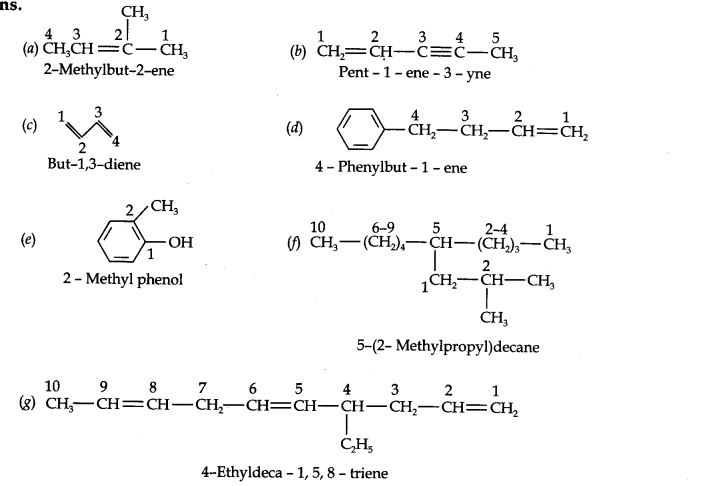
Question 2. Write the IUPAC names of the following compounds:

Answer:
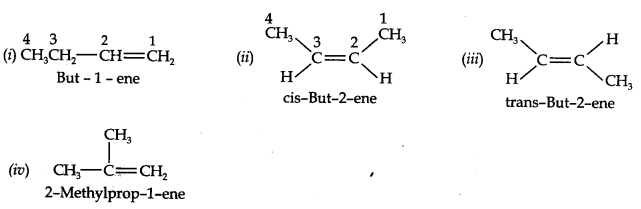
Question 3. For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bonds as indicated:
(a) C4H8 (one double bond) (b) C5H8 (one triple bond)
Answer: (a) Isomers of C4H8 having one double bond are:
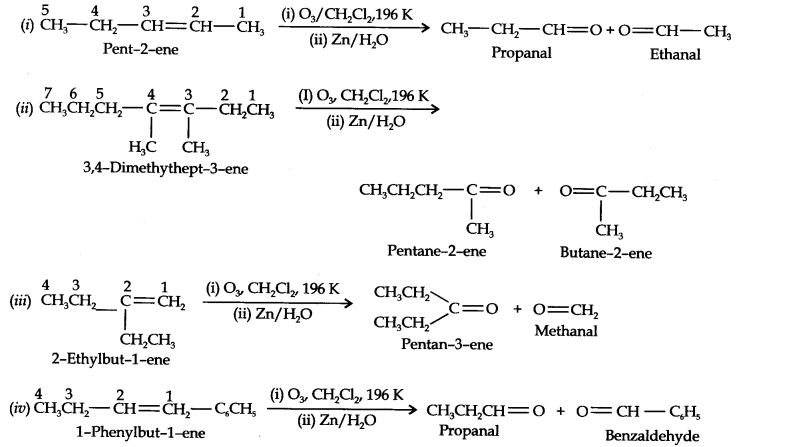
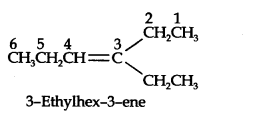
Question 4. Write IUPAC names of the products obtained by the ozonolytic of the following compounds:
(i) Pent-2-ene (ii) 3, 4-Dimethylhept-3-ene (iii) 2-Ethylbut-l-ene (iv) 1-Phenylbut-l-ene.
Answer:
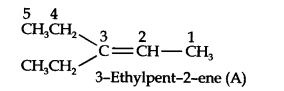
Question 5. An alkene ‘A’ on ozonolysis gives a mixture of ethanol and pentane-3-one. Write the structure and IUPAC name of ‘A’.
Answer: Step 1. Write the structure of the products side by side with their oxygen atoms pointing toward each other.
Step 2. Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene ‘A’ is
Question 6. An alkene ‘A’ contains three C—C, eight C—H, a-bonds, and one C—C n-bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ’A’.
Answer: (i) An aldehyde with a molar mass of 44 u is ethanal, CH3CH=0
(ii) Write two moles of ethanal side by side with their oxygen atoms pointing towards each other.
(iii) Remove the oxygen atoms and join them by a double bond, the structure of alkene ‘A’ is
As required, but-2-ene has three C—C, eight C—H a-bonds, and one C—C π-bond.
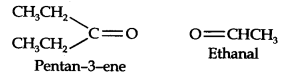
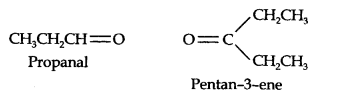
Question 7. Propanal and pentane-3-ene are the ozonolysis products of an alkene. What is the structural formula of the alkene?
Answer: (i) Write the structures of propanal and pentane-3-ene with their oxygen atoms facing each other, we have,
(ii) Remove oxygen atoms and join the two fragments by a double bond, the structure of the alkene is
Question 8. Write chemical equations for the combustion reaction of the following hydrocarbons, (i) Butane (ii) Pentene (iii) Hexyne (iv) Toluene
Answer:
Question 9. Draw the cis- and trans-structures for hex-2-one. Which isomer will have higher b.p. and why?
Answer: The structures of cis- and trans-isomer of hex-2-ene are:
The boiling point of a molecule depends upon dipole-dipole interactions. Since cis-isomer has a higher dipole moment, therefore, it has a higher boiling point.
Question 10. Why is benzene extra-ordinarily stable though it contains three double bonds?
Answer:
The six electrons of the p-orbitals cover all six carbon atoms and are said to be delocalized. As a result of delocalization, there formed a stronger n-bond and a more stable molecule.
Question 11. What are the necessary conditions for any system to be aromatic?
Answer: The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalized n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow a cyclic overlap of p-orbitals.
- It should contain Huckel’s number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3, etc.
A molecule that does not satisfy any one or more of the above conditions is said to be non-aromatic.
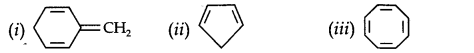
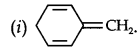
Question 12. Explain why the following systems are not aromatic.

Answer:
Due to the presence of a sp3-hybridized carbon, the system is not
planar. It does contain six n-electrons but the system is not fully conjugated since all the six n-electrons do not form a single cyclic electron cloud that surrounds all the atoms of the ring. Therefore, it is not an aromatic compound.
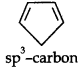
(ii)
Due to the presence of a sp3 -carbon, the system is not planar. Further, it contains only four n-electrons, therefore, the system is not aromatic because it does not contain a planar cyclic cloud having (4n + 2) n-electrons.
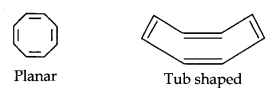
(iii)
Cyclo-octatetraene is not planar but is tub-shaped. It is, therefore, a non-planar system having 8 n-electrons.
Therefore, the molecule is not aromatic since it does not contain a planar cyclic cloud having (4n + 2) n-electrons.
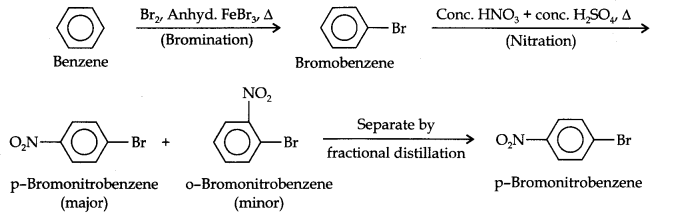
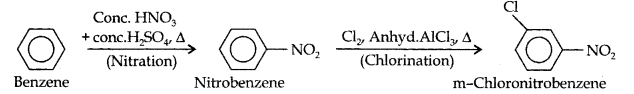
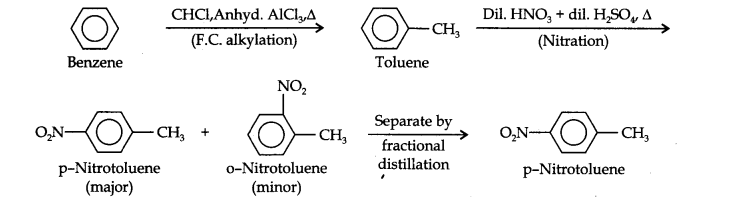
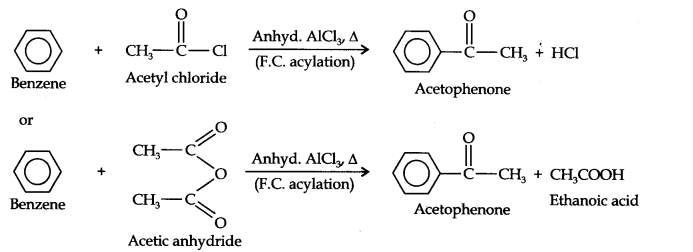
Question 13. How will you convert benzene into (i)p-nitrobromobenzene (ii) m-nitrochlorobenzene (iii) p-nitrotoluene (iv) acetophenone?
Answer: (i) The two substituents in the benzene ring are present at p-positions. Therefore, the sequence of reactions should be such that first an o, p-directing group, i.e., Br atom should be introduced in the benzene ring and this should be followed by nitration. Thus,
(ii) Here since the two substituents are at p-position w.r.t. each other, therefore, the first substituent in the benzene ring should be a o, p-directing group (i.e., CH3) and then the other group (i.e., NO2) should be introduced. Therefore, the sequence of reactions is:
(iii)Here since the two substituents are at m-position w.r.t. each other, therefore, the first substituent in the benzene ring should be a m-directing group (i.e., NO2), and then the other group (i.e., Cl) should be introduced.
(iv)Acetophenone can be prepared by F.C. acylation using either acetyl chloride or acetic anhydride.
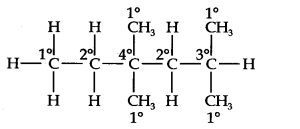
Question 14. In the alkane, CH3CH2—C(CH3)2—CH2—CH(CH3)2, identify 1°, 2°, 3° carbon atoms and give the number of H-atoms bonded to each one of these.
Answer: The expanded formula of the given compound is
Question 15. What effect does the branching of an alkane chain have on its boiling point?
Answer: The branching of the carbon atom chain decreases the boiling point of alkane.
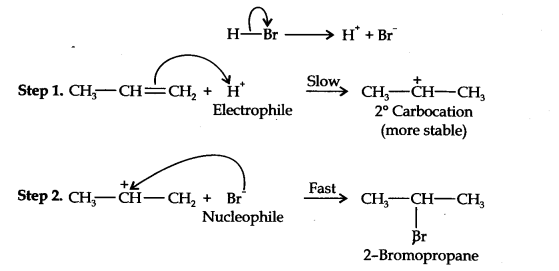
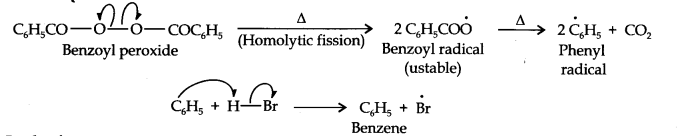
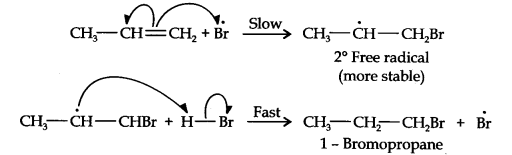
Question 16. Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give the mechanism.
Answer: The addition of HBr to propene is an ionic electrophilic addition reaction in which the electrophile, i.e., H+ first adds to give a more stable 2° carbocation. In the 2nd step, the carbocation is rapidly attacked by the nucleophile Br~ ion to give 2-bromopropane.
In the presence of benzoyl peroxide, the reaction is still electrophilic but the electrophile here is a Br free radical which is obtained by the action of benzoyl peroxide on HBr
In the first step, Br radical adds to propene in such a way so as to generate the more stable 2° free radical. In the second step, the free radical thus obtained rapidly abstracts a hydrogen atom from HBr to give 1-bromopropane.
From the above discussion, it is evident that although both reactions are electrophilic addition reactions it is due to different orders of addition of H and Br atoms which gives different products.
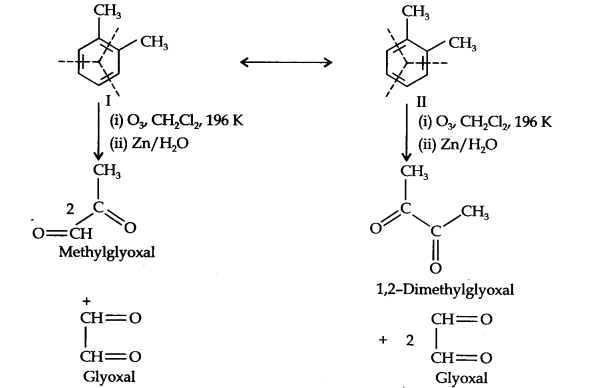
Question 17. Write down the products ozonolysis of, 2-dimethylbenzene (o-xylene). How does the result support the Kekune structure of benzene?
Answer: o-Xylene may be regarded as a resonance hybrid of the following two Kekune structures. Ozonolysis of each one of these gives two products as shown below:
Thus, in all, three products are formed. Since all three products cannot be obtained from any one of the two Kekule structures, this’ shows that o-xylene is a resonance hybrid of the two Kekule structures (I and II).
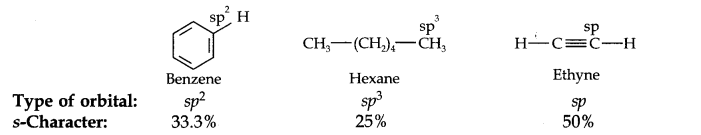
Question 18. Arrange benzene, n-hexane, and ethyne in decreasing order of acidic behavior. Also, give a reason for this behavior.
Answer: The hybridization state of carbon in these three compounds is:
Since s-electrons are closer to the nucleus, therefore, as the s-character of the orbital making the C—H bond increases, the electrons of the C—H bond lie closer and closer to the carbon atom.
In other words, the partial +ve charge on the H-atom and hence the acidic character increases as the s-character of the orbital increases. Thus, the acidic character decreases in the order: Ethyne > Benzene > Hexane.
Question 19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer: Due to the presence of an electron cloud containing 6 n-electrons above and below the plane of the ring, benzene is a rich source of electrons.
Consequently, it attracts the electrophiles (electron-deficient) reagents towards it and repels nucleophiles (electron-rich) reagents. As a result, benzene undergoes electrophilic substitution reactions easily and nucleophilic substitutions with difficulty.
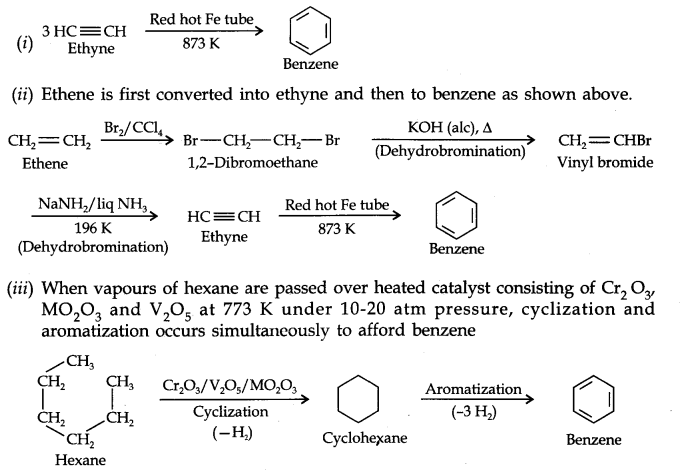
Question 20. How will you convert the following compounds into benzene?
(i) Ethyne (ii) Ethene (iii) Hexane.
Answer:
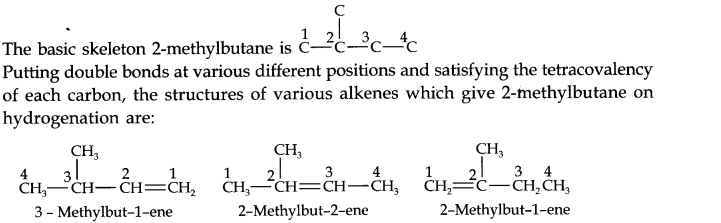
Question 21. Write structures of all the alkenes which on hydrogeneration give 2-methylbutane.
Answer:
Question 22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+.
(a) Chlorobenzene, 2, 4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene—H3C—C6H4—NO2, p—O2N—C6H4—NO2.
Answer: (a) The typical reactions of benzene are electrophilic substitution reactions. The higher the electron density in the benzene ring, the more reactive the compound is toward these reactions. Since N02 is a more powerful electron-withdrawing group than Cl, therefore, more the number of nitro groups, the less reactive the compound. Thus, the overall reactivity decreases in the order:
Chlorobenzene > p-nitrochlorobenzene > 2, 4-dinitrochlorobenzene
(b) Here, the CH3 group is electron donating but the N02 group is electron-withdrawing. Therefore, the maximum electron density will be in toluene, followed by p-nitrotoluene followed by p-dinitrobenzene. Thus, the overall reactivity decreases in the order:
Toluene >p—H3C—C6H4—NO2, p—O2N—C6H4—NO2.
Question 23. Out of benzene, m-dinitrobenzene, and toluene which will undergo nitration most easily and why?
Answer: CH3 group is electron-donating while—NO2 group is electron-withdrawing. Therefore, the maximum electron density will be in toluene, followed by benzene, and at least in m-dinitrobenzene. Therefore, the ease of nitration decreases in the order: toluene > benzene > m-dinitrobenzene.
Question 24. Suggest the name of another Lewis acid instead of anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer: Anhydrous Ferric Chloride (FeCl3) is another Lewis acid that can be used.
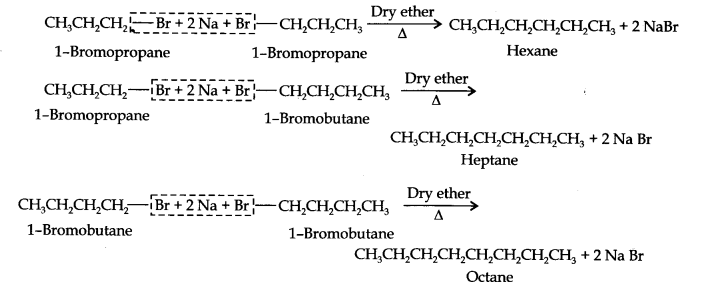
Question 25. Why is the Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms? Illustrate your answer by taking one example.
Answer: For the preparation of alkanes containing an odd number of carbon atoms, a mixture of two alkyl halides has to be used. Since two alkyl halides can react in three different ways, therefore, a mixture of three alkanes instead of the desired alkane would be formed.
For example, the Wurtz reaction between ‘1-bromopropane and 1-bromobutane gives a mixture of three alkanes i.e., hexane, heptane, and octane as shown below:
MORE QUESTIONS SOLVED
NCERT Solutions for Class 11 Chemistry Chapter 13 Very Short Answer Type Questions
Question 1.
 >
>
Answer:
Question 2. What are conformations?
Answer: Conformations are spatial arrangements that are obtained by rotation around sigma bonds.
Question 3. What is decarboxylation? Give an example.
Answer: The process by which carbon dioxide is removed from sodium acetate (or any sodium salt of acid) with the help of soda lime is called decarboxylation.
Question 4. What do you mean by pyrolysis?
Answer: The decomposition of a compound by heat is called pyrolysis. This process when applied to alkanes is known as cracking.
Question 5. What happens when ethanol is heated with a cone? H2SO4?
Answer:
Question 6. Convert ethylene to ethane.
Answer:
Question 7. What is Lindlar’s catalyst? Give its use.
Answer: Pd/BaSO4 is known as Lindlar’s catalyst. It is used in the conversion of alkynes to alkenes with the help of H2.
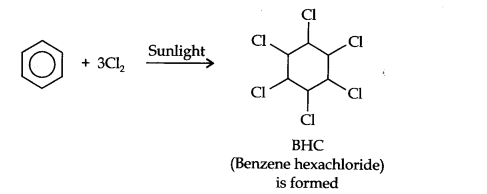
Question 8. What happens when benzene is treated with an excess of Cl2 in the presence of sunlight? Give chemical reaction.
Answer:
Question 9. Why are alkanes called paraffin?
Answer: Paraffin means little affinity. Alkanes due to strong C—C and C—H bonds are relatively chemically inert.
Question 10. Arrange the three isomers of pentane in increasing order of their boiling points.
Answer: 2, 2-Dimethylpropane < 2-methyl butane < pentane.
Question 11. Arrange the following: HCl, HBr, HI, HF in order of decreasing reactivity towards alkenes.
Answer: HI > HBr > HCl > HF
Question 12. Although benzene is highly unsaturated it does not undergo addition reactions. Why?
Answer: It is due to the delocalization of π -electrons in benzene it is highly stable.
Question 13. Why are Alkenes called olefins?
Answer: Alkenes are commonly known as olefins because the lower members form oily products on treatment with chlorine or bromine.
Question 14. Which is more acidic: ethene or ethyne and why?
Answer: Ethyne is more acidic than ethene because it has ‘sp’ hybridized ‘C’ which is more electronegative.
Question 15. What is Huckel’s rule?
Answer: Huckel rule states that a compound is said to be aromatic if it has (4n + 2) n electrons
delocalized where n = an integer 0,1, 2, 3,
Question 16. How will you distinguish between propene and propane?
Answer: Pass them through a dilute cold KMnO4 solution (purple) or Br2 in CCl4 solution (red). Propene will decolorize both the solutions but propane does not react.
Question 17. How will you distinguish between acetylene and ethylene?
Answer: Acetylene forms a precipitate with ammoniacal silver nitrate solution, ethylene does not react with these reagents.
Question 18. What happens when benzene is treated with acetyl chloride in the presence of AlCl3?
Answer: Acetophenone is formed.
Question 19. Which type of isomerism is exhibited by but-l-one and but-2-one?
Answer: Position isomerism.
Question 20. What is electrophile in sulphonation?
Answer: SO3.
Question 21. What is the hybridization of central carbon in 1,2-propadiene (CH2=C=CH2)?
Answer: sp.
Question 22. What are Arenes?
Answer: Arenes are aromatic hydrocarbons.
We have covered the complete guide on CBSE NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Structure Of Atom. Feel free to ask us any questions in the comment section below.
FAQ: NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Structure Of Atom
Can I download NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Structure Of Atom PDF for free?
Yes, you can download NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons Structure Of Atom PDF free.
What are aromatic hydrocarbons?
Those hydrocarbons formed by Benzene and its derivatives are known as aromatic hydrocarbons.
What are the Benefits of Studying from NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
You can refer to the above article to get the Benefits of Studying from NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons.
What will I learn in NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons?
You can refer to the above article to know the learnings of NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons.
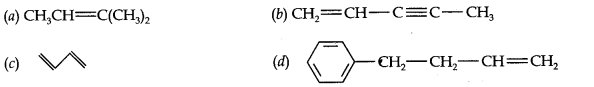
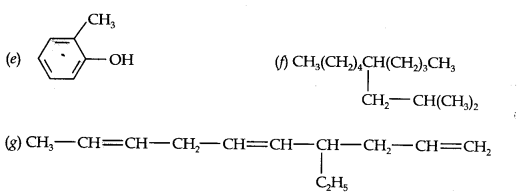
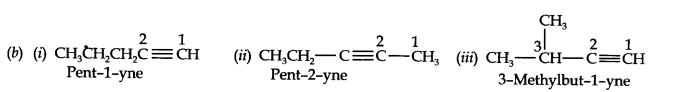
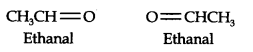
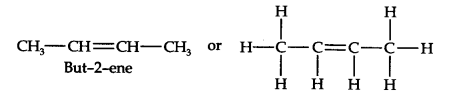
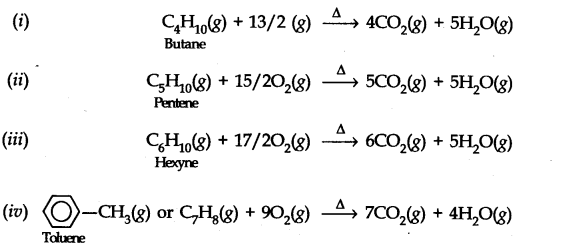
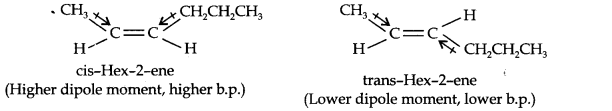
 >
>