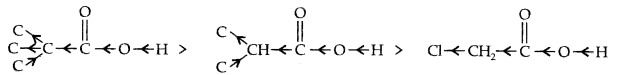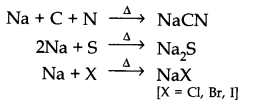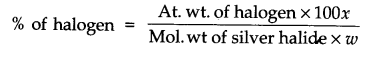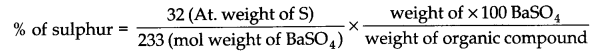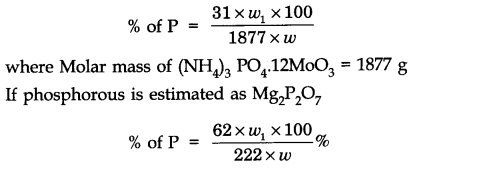NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry: Chemistry is an essential subject for class 11. And chapter 12, which deals with the basic concepts in organic chemistry, is one of the necessary topics that help build a solid foundation in the subject. So, you get NCERT Solutions for Class 11 Chemistry Chapter 12 for the same.
And by going over them, you can come out in flying colors in not only your 11th-grade final exams but also in your 12th-grade board exams as well as in various other competitive exams such as NEET and IIT-JEE. The solutions are comprehensive, and they explain each concept in detail. So, you should be studying from them if you ought to crack these exams.
NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry
As a student of class 11 looking to put in your best efforts, then you should be studying from NCERT solutions. The solutions not only explain each concept in detail but also have questions at the end of every chapter. And by answering them, you can rest assured that you will score higher in the exams.
Also, each chapter in the solutions is according to the topics that you can find in the textbooks. So, it makes for easy navigation.
Organic Chemistry is an essential concept in the subject. And once you have gone through the chapter, you can understand the mechanism underlying organic reactions and name different compounds according to the IUPAC system while also deriving their structures.
Besides this, you will also learn about the different processes behind the purification of organic compounds, as well as how you can interpret them.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry from below.
Download NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2020/09/chapter_12_organic_chemistry_some_basic_principles.pdf”, “#example1”);
What will you learn in CBSE Class 11 Chemistry Chapter 12 Organic Chemistry?
Organic Chemistry is a branch of science and chemistry that deals with organic compounds. Organic compounds are those substances that you can extract from plants and other living beings. Organic Chemistry is the study of carbon compounds and their derivatives. The current chapter is all about organic compounds such as carbon, their shapes and sizes, and their naming, classification, and other such fundamental concepts.
Introduction to Organic Chemistry
Organic Chemistry plays a vital role in everyday life. All the foods, medicines, soaps, perfumes, and other such things that we use are organic compounds. The field of chemistry comes in handy, especially for pharmaceutical professionals who deal with the manufacturing of such compounds.
Catenation
Organic compounds get their shape with the help of a process called ‘catenation.’ In this process, different compounds automatically link with each other to form two-dimensional layers or three-dimensional lattices.
Representation of Organic Compounds
You can represent the structures of carbon-based organic compounds using three methods. The first method, called ‘complete structural formula,’ shows all the atoms and molecules, along with the types of molecules that help create bonds between them. The second technique of showing the structures of organic compounds, called ‘condensed structural formula,’ makes use of abbreviations to save space. Similarly, the third type, also known as the ‘Bond Line Structural Formula,’ is even more condensed than the condensed structural formula. However, when representing the structures of organic compounds using the ‘Bond Line Structural Formula,’ the reader must visualize the things that are left out to get the complete picture.
Classification of Organic Compounds
Organic compounds are classified depending on the number of chains that the carbon bonds form. For example, acyclic compounds consist of both open-chain bonds and closed-chain bonds and are also called Aliphatic Compounds. Similarly, you have aromatic compounds, which are mostly benzene-ring compounds. You also get heterocyclic aromatic compounds produced in a laboratory setting.
The IUPAC creates the rules for naming organic compounds. And the nomenclature depends on the arrangement of the molecules in an organic compound.
The chapter also introduces the students to the concept of ‘Isomerism.’ Isomerism is a phenomenon by which two organic compounds have the same chemical formula but different structures. There are two types of Isomerism. In the first type, called ‘Structural Isomerism,’ two molecules have the same molecular formula. Whereas, in the second type, called ‘Stereo Isomerism,’ they would have the same molecular formula, but different three-dimensional orientations.
Organic Compounds and their Reactions
Organic compounds react depending on three factors, namely, their shape, the functional group that they belong to, and how they differ from each other in terms of the previous one in a series.
Purification of Organic Compounds
Organic compounds can be purified to get pure compounds using eight different techniques. A few crucial processes involved in all the eight procedures include distillation, crystallization, sublimation, and chromatography.
Advantages of NCERT Solutions 11th Chemistry Chapter 12 Organic Chemistry
Studying from NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry can be beneficial, especially for a subject like Chemistry. The solutions contain a detailed, chapter-wise explanation of the concepts. So, students can be thorough with each of the topics by going through them. Likewise, here are the other advantages of studying organic chemistry from NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry.
1. Clear and In-Depth Analysis of the Concepts
NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry give a clear picture of the different concepts in organic chemistry along with in-depth explanations for the same.
2. Easy to Navigate
The solutions come in a chapter-wise arrangement, just like the textbooks. And the creators have broken down each chapter into the different concepts. So, it makes for easy navigation.
3. A Guide to Preparing for Competitive Exams
NCERT solutions help students prepare themselves for various competitive examinations such as the IIT-JEE or NEET. They give those questions that are vital for such exams at the end of each chapter. So, students can prepare to face competitive exams with confidence.
NCERT Solutions for Chemistry, and mainly, for a vast and varied concept like Organic Chemistry, can be helpful for students who aspire to write exams like the IIT-JEE, NEET, AIIMS, and other such competitive exams. Additionally, they also help them prepare for the upcoming class 11 exams as well as the board exams of grade 12. So, there is no doubt that NCERT solutions are one of the best study materials when it comes to class 11 Chemistry.
Access NCERT Solutions For Class 11 Chemistry Chapter 12
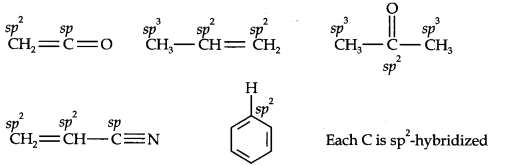
Question 1. What are hybridisation states of each carbon atom in the following compounds? CH2=C=O, CH3CH=CH2, (CH3)2CO, CH2=CHCN, C6H6.
Answer:
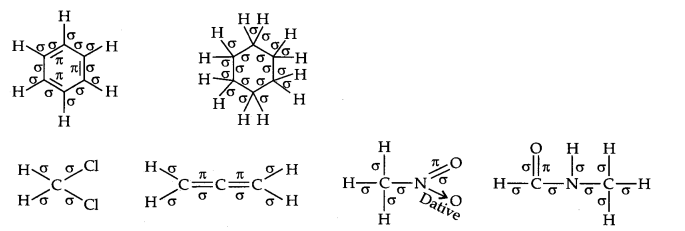
Question 2. Indicate the a- and n-bonds in the following molecules:
C6H6 , C6H12, CH2Cl2, CH=C=CH2, CH3NO2, HCONHCH3
Answer:
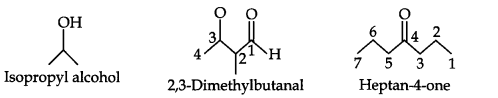
Question 3. Write bond-line formulas for: Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one.
Answer:
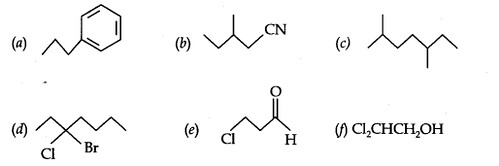
Question 4. Give the TUPAC names of the following compounds:

Answer: (a) Propyl benzene (b) 3-Methylpentanenitrite (c) 2, 5-Dimethylheptane
(d) 3-Bromo- 3-chloroheptane (e) 3-Chloropropanal (f) 2, 2-Dichloroethanol
Question 5.Which of the following represents the correct TUPAC name for the compounds concerned?
(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyl octane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- l-ol or But-4-ol-yne.
Answer: (a) 2, 2-Demethylpentane (b)2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7.
(c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-l-ol. Lower locant for the principal functional group, i.e., alcohol.
Question 6. Draw formulas for the first five members of each homologous series beginning with the following compounds,
(a) H—COOH (b) CH3COCH3 (c) H—CH=CH2
Answer: (a) CH3—COOH
CH3CH2—COOH CH3CH2CH2—COOH
CH3CH2CH2CH2—COOH
(b) CH3COCH3
CH3COCH2CH3
CH3COCH2CH2CH3
CH3COCH2CH2CH2CH3
CH3CO(CH3)4CH3
(c) H—CH=CH2
CH3CH=CH2
CH3CH2CH=CH2
CH3CH2CH2CH=CH2
CH3CH2CH2CH2CH=CH2
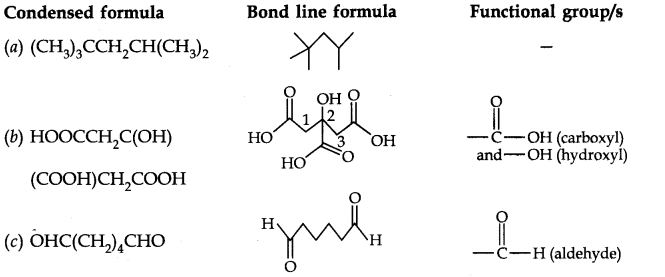
Question 7. Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for: (a) 2, 2, 4-Trimethylpentane (b) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid (c) Hexanedial.
Answer:
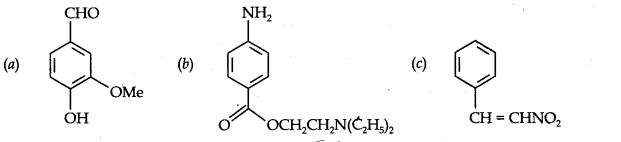
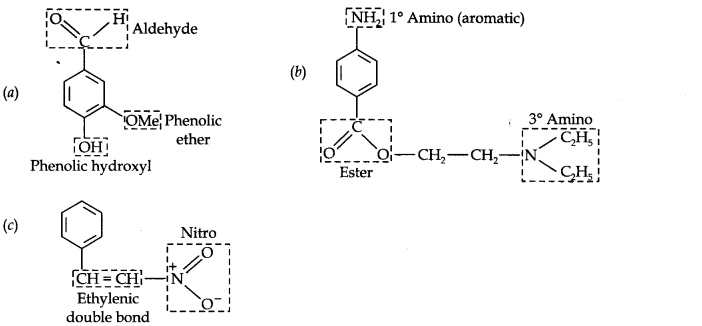
Question 8. Identify the functional groups in the following compounds:

Answer:
Question 9. Which of the two: O2NCH2CH2O– or CH3CH2O– is expected to be more stable and why?
Answer: O2N——<——- CH2——<——- CH2 —<——- O– is more stable than CH3——<——-CH2——<——-O- because NO2 group has -I-effect and hence it tends to disperse the -ve charge on the O-atom. In contrast, CH3CH2 has +I-effect. It, therefore, tends to intensify the -ve charge and hence destabilizes it.
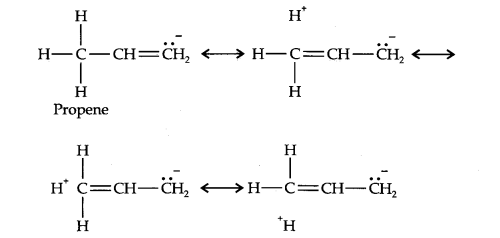
Question 10. Explain why alkyl groups act as electron donors when attached to a π-system.
Answer: Due to hyperconjugation, alkyl groups act as electron donors when attached to a π- system as shown below:
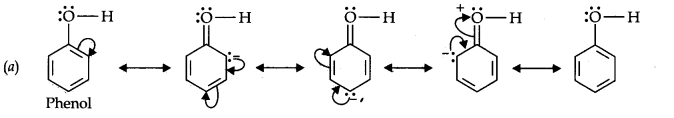
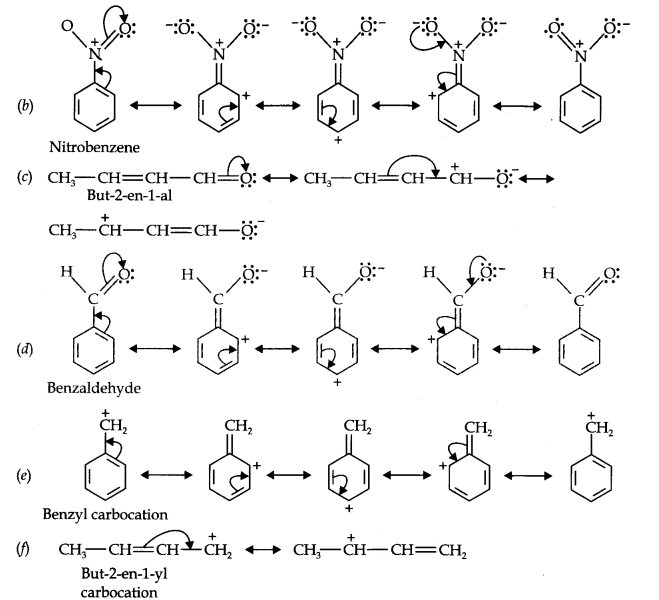
Question 11. Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C6H5OH (b) C6H5N02 (c) CH3CH=CHCHO (d) C6H5—CHO (e) C6H5—CH2 (f) Ch3Ch=ChCh2
Answer:
Question 12. What are electrophiles and nucleophiles? Explain with examples:
Answer: Electrophiles: The name electrophiles means electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules.
Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3
Nucleophiles: The name nucleophiles means ‘nucleus loving’ and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule.
They are electron rich they may be negative ions or neutral molecules.
Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
Question 13. Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles
(a) CH3COOH + HO– ———–> CH3COO– + H2O
(b) CH3COCH3 + CN ———–> (CH3)2 C(CN)(OH)
(c) C6H5 + CH3CO ———–> C6H5COCH3
Answer: Nucleophiles: (a) and (b) and Electrophile : (c)
Question 14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– ———–> CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl ———–> (CH3)2CCl—CH3
(c) CH3CH2Br + HO– ———–> CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr ———–> (CH3)2 C Br CH2CH2CH3 + H2O
Answer: (a) Nucleophilic substitution (b) Electrophilic addition
(c)Bimolecular elimination (d) Nucleophilic substitution with rearrangement.
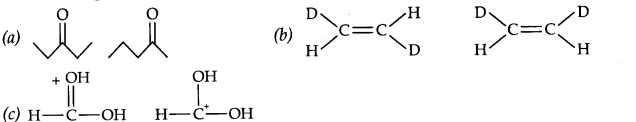
Question 15. What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?

Answer: (a) Structural isomers (actually position isomers as well as metamers)
(b) geometrical isomers
(c) resonance contributors because they differ in the position of electrons but not atoms
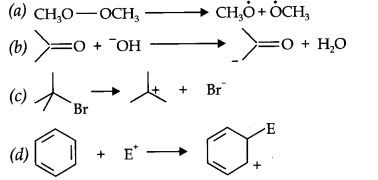
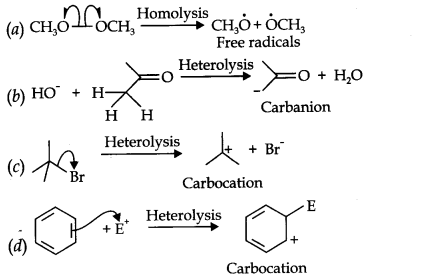
Question 16. For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis.
Identify reactive intermediate produced as free radical, carbocation and carbanion.

Answer:
Question 17. Explain the terms inductive and electrometric effects. Which electron displacement effect explain the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
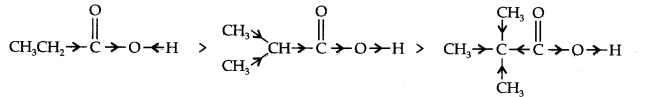
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
Answer: Inductive Effect: The inductive effect refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another. Atoms or groups which lose electron towards a carbon atom are said to have +1 Effect.
Those atoms or groups which draw electron away from a carbon atom are said to have -I Effect.
Common examples of -I effect are:
NO2, F, Cl, Br, I, OH etc.
Examples of +1 effect are (Electron releasing)
(CH3)2C— , (CH3)2CH—, CH3CH2— CH3— etc.
Electrometric effect: The electrometric effect refers to the polarity produced in a multiple bonded compound as it is approached by a reagent.
The atom A has lost its share in the electron pair and B has gained this share.
As a result A acquires a positive charge and B a negative charge. It is a temporary effect and takes place only in the presence of a reagent.
(a) -I-effect as shown below:
As the number of halogen atoms decreases, the overall -I- effect decreases and the acid strength decreases accordingly.
(b) +I-effect as shown below:
As the number of alkyl groups increases, the +I-effect increases and the acid strength
decreases accordingly.
Question 18. Give a brief description of the principles of the following techniques taking an example in each case: (a) Crystallisation (b) Distillation (c) Chromatography
Answer: (a) Crystallisation: In this process the impure solid is dissolved in the minimum volume of a suitable solvent. The soluble impurities pass into the solution while the insoluble ones left behind.
The hot solution is then filtered and allowed to cool undisturbed till crystallisation is complete. The crystals are then separated from the mother liquor by filtraration and dried.
Example: crystallisation of sugar.
(b) Distillation: The operation of distillation is employed for the purification of liquids from non-volatile impurities. The impure liquid is boiled in a flask and the vapors so formed are collected and condensed to give back pure liquid in another vessel. Simple organic liquids such as benzene toluene, xylene etc. can be purified.
(c) Chromatography: Chromatography is based on the principle of selective distribution of the components of a mixture between two phases, a stationary phase and a moving phase. The stationary phase can be a solid or liquid, while the moving phase is a liquid or a gas.
When the stationary phase is solid the basis is adsorption and when it is a liquid the basis is partition. Chromatography is generally used for the Reparation of coloured substances such as plant pigments or dyestuffs.
Question 19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Answer: Fractional crystallisation is used for this purpose. A hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out while the more soluble remains in the solution.
The crystals are separated from the mother liquor and the mother liquor is again concentrated and the hot solution again allowed to cool when the crystals of the second compound are obtained. These are again filtered and dried.
Question 20. What is the difference between distillation, distillation under reduced pressure and steam distillation?
Answer: Distillation is used in case of volatile liquid mixed with non-volatile impurities.
Distillation under reduced pressure: This method is used to purify such liquids which have very high boiling points and which decompose at or below their boiling points.
Steam distillation is used to purify steam volatile liquids associated with water immiscible impuritites.
Question 21. Discuss the chemistry of Lassaigne’s test.
Answer: Lassaigne’s test: Nitrogen, sulphur, halogens and phosphorous present in an organic compound are detected by Lassaigne’s test.
First of all compounds are converted to ionic form by fusing the compound with sodium metal.
Cyanide, sulphide or halide of sodium are extracted from the fused mass by boiling it with distilled water. This extract is known as sodium fusion extract.
Question 22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method.
Answer: (i) Dumas method: The organic compound is heated strongly with excess of CuO ‘ (Cupric Oxide) in an atmosphere of CO2 when free nitrogen, CO2 and H2O are obtained.
(ii)Kjeldahl’s method: A known mass of the organic compound is heated strongly with cone. H2SO4, a little potassium sulphate and a little mercury (a catalyst). As a result of reaction the nitrogen present in the organic compound is converted to ammonium sulphate.
Question 23. Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Answer: Estimation of halogens: It involves oxidising the organic substance with fuming nitric acid in the presence of silver nitrate. The halogen of the substance is thus converted to silver halide which is separated and weighed:
1Weight of organic compound = W gm
weight of silver halide = x g.
Estimation of sulphur: The organic substance is heated with fuming nitric acid but no silver nitrate is added. The sulphur of the substance is oxidized to sulphur acid which is then precipitated as barium sulphate by adding excess of barium chloride solution. From the weight of BaSO4 so obtained the percentage of sulphur can be calculated.
Estimation of phosphorous: The organic substance is heated with fuming nitric acid whereupon phosphorous is oxidised to phosphoric acid. The phosphoric acid is precipitated as ammonium phosphomolybdate, (NH4)3 PO4 .12MOO3, by the addition of ammonia and ammonium molybdate solution which is then separated, dried and weighed.
Question 24. Explain the principle of paper chromatography.
Answer: This is the simplest form of chromatography. Here a strip of paper acts as an adsorbent. It is based on the principle which is partly adsorption. The paper is made of cellulose fibers with molecules of water adsorbed on them.
This acts as stationary phase. The mobile phase is the mixture of the components to be identified prepared in a suitable solvent.
Question 25. Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens ?
Answer: Nitric acid is added to sodium extract so as to decompose
NaCN + HNO3 ——-> NaNO3 + HCN
Na2S + 2HNO3 ——> 2NaNO3 + H2S
Question 26. Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Answer: Organic compound is fused with sodium metal so as to convert organic compounds into NaCN, Na2S, NaX and Na3PO4. Since these are ionic compounds and become more reactive and thus can be easily tested by suitable reagents.
Question 27. Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Answer: Sublimation. Because camphor can sublime whereas CaSO4 does not.
Question 28. Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation ?
Answer: It is because in steam distillation the sum of vapor pressure of organic compound and steam should be equal to atmospheric pressure.
Question 29.Will CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer.
Answer: No. CCl4 is a completely non-polar covalent compound whereas AgNO3 is ionic in nature. Therefore they are not expected to react and thus a white ppt. of silver chloride will not be formed.
Question 30. Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound?
Answer: CO2 is acidic in nature and therefore, it reacts with the strong base KOH to form K2CO3.
2KOH + CO2 ——–> K2CO3+ H2O.
We have covered the complete guide on CBSE NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry. Feel free to ask us any questions in the comment section below.
FAQ: NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry
How many chapters are there in chemistry NCERT Solutions for Class 11 Chemistry Chapter 12 organic chemistry?
There are 14 chapters in NCERT Solutions for Class 11 Chemistry Chapter 12 combining both Part 1 & Part 2.
What are the basic concepts of organic chemistry?
Organic chemistry is the study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds, which include not only hydrocarbons but also compounds with any number of other elements, including hydrogen, nitrogen, oxygen, halogens, phosphorus, silicon, and sulphur.
Is NCERT Solutions for Class 11 Chemistry Chapter 12 chemistry hard?
It is one of the easiest but highly scoring chapters of NCERT Solutions for Class 11 Chemistry Chapter 12.
Is organic chemistry hard?
Organic chemistry is the most dreaded of all science classes. It has the highest failure rate, lowest class average, and more retakes than any other science course.